SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 1069.0 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 24 |
| Rotatable Bond Count | 26 |
| Exact Mass | 1068.39836869 g/mol |
| Monoisotopic Mass | 1068.39836869 g/mol |
| Topological Polar Surface Area | 362 Ų |
| Heavy Atom Count | 74 |
| Formal Charge | 0 |
| Complexity | 799 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
Description
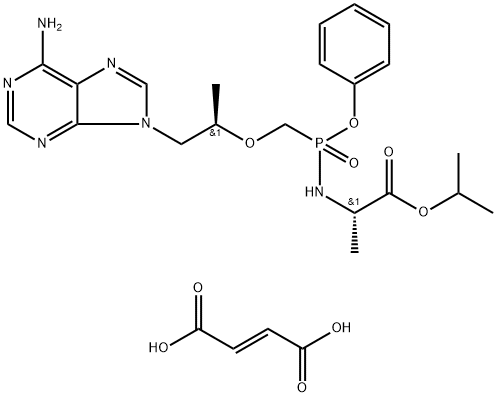
Tenofovir alafenamide hemifumarate is a fumarate salt prepared from tenofovir alafenamide by reaction of one molecule of fumaric acid for every two molecules of tenofovir alafenamide. A prodrug for tenofovir, it is used in combination therapy for the treatment of HIV-1 infection. It has a role as an antiviral drug, an HIV-1 reverse transcriptase inhibitor and a prodrug.