COMPUTED DESCRIPTORS
| Molecular Weight | 297.14 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 297.0842240 g/mol |
| Monoisotopic Mass | 297.0842240 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 370 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
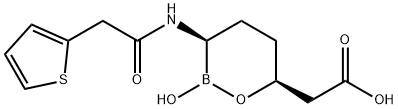
Vaborbactam is a β-lactamase inhibitor based on a cyclic boronic acid pharmacophore. It has been used in trials investigating the treatment of bacterial infections in subjects with varying degrees of renal insufficiency. In August 2017, a combination antibacterial therapy under the market name Vabomere was approved by the FDA for the treatment of adult patients with complicated urinary tract infections (cUTI). Vabomere consists of vaborbactam and [Meropenem] for intravenous administration. Vaborbactam is added to the therapy to reduce the extent meropenem degradation by inhibiting the serine beta-lactamases expressed by the microorganism of target. The treatment aims to resolve infection-related symptoms of cUTI and achieve negative urine culture, when the infections are proven or strongly suspected to be caused by susceptible bacteria.