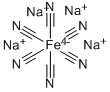
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium ferrocyanide is an odorless yellow solid. Sinks and mixes with water. (USCG, 1999) |
|---|---|
| Color/Form | Pale yellow |
| Odor | ODORLESS |
| Solubility | Solubility (% in water): 10.2% @ 1 °C; 14.7% @ 17 °C; 17.6% @ 25 °C; 28.1% @ 53 °C; 39% @ 85 °C; 39.7% @ 96.6 °C /Calculated as anhydrous/ |
| Density | 1.458 at 77 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | SLIGHTLY EFFLORESCENT; STEADY DEHYDRATION OCCURS ABOVE 50 °C /DECAHYDRATE/ |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | WHEN HEATED TO DECOMP OR ON CONTACT WITH ACID OR ACID FUMES, THEY EMIT HIGHLY TOXIC FUMES OF CYANIDES. /FERROCYANIDES/ |
| Other Experimental Properties | SOLUBILITY (G/100 CC WATER): 31.85 G @ 20 °C, 156.5 G @ 98 °C; INDEX OF REFRACTION: 1.519, 1.530, 1.544; DENSITY 1.458 /DECAHYDRATE/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 303.91 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 0 |
| Exact Mass | 303.912457 g/mol |
| Monoisotopic Mass | 303.912457 g/mol |
| Topological Polar Surface Area | 143 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 127 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 11 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium ferrocyanide is an odorless yellow solid. Sinks and mixes with water. (USCG, 1999)