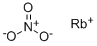CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White crystalline powder; [Acros Organics MSDS] |
|---|---|
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 147.473 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 146.89960760 g/mol |
| Monoisotopic Mass | 146.89960760 g/mol |
| Topological Polar Surface Area | 62.9 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Rubidium nitrate is a nitrate of rubidium. It is used in pyrotechnic compositions as a colorant and an oxidizer. Nitrite is a toxic compound known to cause methemoglobinemia. (L1137, L1635)