SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 507.5 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 8 |
| Exact Mass | 507.07298423 g/mol |
| Monoisotopic Mass | 507.07298423 g/mol |
| Topological Polar Surface Area | 241 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 861 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
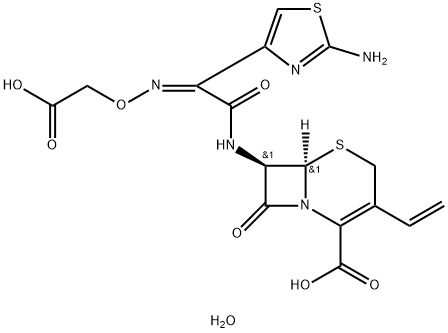
description
Cefixime is a broad-spectrum, third-generation cephalosporin antibiotic derived semisynthetically from the marine fungus Cephalosporium acremonium with antibacterial activity. As does penicillin, the beta-lactam antibiotic cefixime inhibits bacterial cell wall synthesis by disrupting peptidoglycan synthesis, resulting in a reduction in bacterial cell wall stability and bacterial cell lysis. Stable in the presence of a variety of beta-lactamases, this agent is more active against gram-negative bacteria and less active against gram-positive bacteria compared to second-generation cephalosporins.