123-08-0
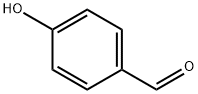
Product Name:
4-Hydroxybenzaldehyde
Formula:
C7H6O2
Synonyms:
p-Hydroxybenzaldehyde;4-Hydroxybenzaldehyde
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; Sublimes at atmospheric pressure without decomposition; [Merck Index] Light brown crystalline solid; [Aldrich MSDS] |
|---|---|
| Boiling Point | 310 °C |
| Melting Point | 117 °C |
| Solubility | 8450 mg/L (at 25 °C) |
| LogP | 1.35 |
| LogS | -0.96 |
| Ionization Efficiency | Negative |
| Dissociation Constants | 7.61 (at 25 °C) |
| Collision Cross Section | 118.2 Ų [M-H]- [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)] |
| Kovats Retention Index | 1311 1317 1308 1318 1324 1318.6 1320.8 1320 1302 1318 1320 1323 1320 1313.5 |
| Chemical Classes | Other Classes -> Benzaldehydes |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 122.12 g/mol |
|---|---|
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 122.036779430 g/mol |
| Monoisotopic Mass | 122.036779430 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 93.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
4-hydroxybenzaldehyde is a hydroxybenzaldehyde that is benzaldehyde substituted with a hydroxy group at position C-4. It has a role as a plant metabolite, a mouse metabolite and an EC 1.14.17.1 (dopamine beta-monooxygenase) inhibitor.
