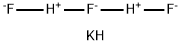CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Potassium fluoride appears as white powder or crystals with a sharp saline taste. Shipped as a solid or an aqueous solution. Strongly irritates skin, eyes and mucous membranes. Toxic by ingestion. Used in insecticides, solder flux and etching glass. |
|---|---|
| Color/Form | White cubic crystals |
| Taste | Sharp saline taste |
| Boiling Point | 2741 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 1576 °F (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 66 °F (NTP, 1992) |
| Density | 2.48 (NTP, 1992) - Denser than water; will sink |
| Vapor Pressure | 100 Pa (0.75 mm Hg) at 869 °C; 1 kPa (7.5 mm Hg) at 1017 °C; 10 kPa (75 mm Hg) at 1216 °C; 100 kPa (750 mm Hg) at 1499 °C |
| Decomposition | When heated to decomposition it emits toxic fumes of /potassium oxide and hydrogen fluoride/. |
| Corrosivity | Aqueous solution corrodes glass and porcelain |
| Other Experimental Properties | May be stored in aluminum containers, attracts moisture from the air |
| Chemical Classes | Other Classes -> Fluorides, Inorganic |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 58.0967 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 57.96210965 g/mol |
| Monoisotopic Mass | 57.96210965 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Potassium fluoride appears as white powder or crystals with a sharp saline taste. Shipped as a solid or an aqueous solution. Strongly irritates skin, eyes and mucous membranes. Toxic by ingestion. Used in insecticides, solder flux and etching glass.