CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Slightly yellow to yellow powder |
|---|---|
| Melting Point | approximately 150 °C |
| Other Experimental Properties | Hygroscopic |
COMPUTED DESCRIPTORS
| Molecular Weight | 690.8 g/mol |
|---|---|
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 7 |
| Exact Mass | 690.32985036 g/mol |
| Monoisotopic Mass | 690.32985036 g/mol |
| Topological Polar Surface Area | 185 Ų |
| Heavy Atom Count | 48 |
| Formal Charge | 0 |
| Complexity | 1340 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
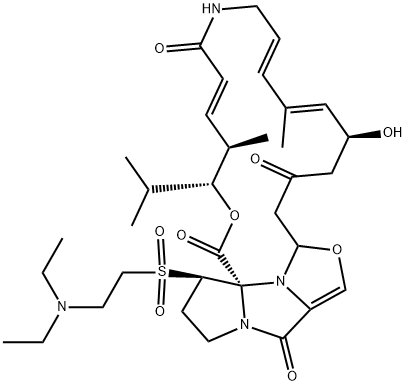
Dalfopristin is a macrolide that is pristinamycin IIA in which the double bond between positions 26 and 26a of the pyrroline ring has been reduced and position 26R carries a [2-(diethylamino)ethyl]sulfonyl group. It is a semi-synthetic streptogramin antibiotic and often used as a mixture with quinupristin for the treatment of vancomycin-resistant Enterococcus faecium. It has a role as an antibacterial drug and a protein synthesis inhibitor. It is a macrolide, a member of 1,3-oxazoles, a cyclic ketone, an enamide, a secondary alcohol, a secondary carboxamide, a tertiary carboxamide, a sulfone, a tertiary amino compound, a carboxylic ester and a macrolide antibiotic. It is functionally related to a pristinamycin IIA.