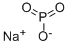CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White solid, practically insoluble in water; [Merck Index] |
|---|---|
| Solubility | White powder. Practically insol in water and in aq solns of pyrophosphates and hexametaphosphates. Sol in mineral acids. Does not form complexes with Fe(II), Fe(III), U(IV) salts. /Maddrell's salt (polymeric sodium metaphosphate)/ |
| Decomposition | When heated to decomposition it emits toxic fumes of sodium oxide and phosphoxides. |
| Other Experimental Properties | Clear, hygroscopic glass. MP: 628 °C. Sol in water, but dissolves slowly. Depolymerizes in aqueous soln to form sodium trimetaphosphate and sodium orthophosphates. /Glassy sodium metaphosphate; Graham's salt/ |
| Chemical Classes | Metals -> Phosphates, Metallic Salts |
COMPUTED DESCRIPTORS
| Molecular Weight | 101.962 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 101.94827514 g/mol |
| Monoisotopic Mass | 101.94827514 g/mol |
| Topological Polar Surface Area | 57.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 49.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | No |