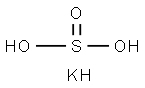
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid |
|---|---|
| Solubility | White crystals or crystalline powder; sol in about 3.5 parts water; decomposed by diluted acids with evolution of sulfur dioxide; pH about 8 /dihydrate/ |
| Stability/Shelf Life | GRADUALLY OXIDIZES IN AIR TO SULFATE. /DIHYDRATE/ |
| Other Experimental Properties | Decomposes on heating /dihydrate/ |
| Chemical Classes | Other Classes -> Sulfites |
SAFETY INFORMATION
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 158.26 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 157.88422800 g/mol |
| Monoisotopic Mass | 157.88422800 g/mol |
| Topological Polar Surface Area | 82.4 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |