CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid |
|---|---|
| Color/Form | Yellow, oily liquid; explodes |
| Odor | Pungent |
| Boiling Point | 71 °C |
| Melting Point | -40 °C |
| Solubility | Insoluble in water |
| Density | 1.653 g/cu cm |
| Vapor Pressure | VP: 1 kPa at -25 °C (ext); 10 kPa at 13.2 °C; 100 kPa at 70.6 °C |
| Stability/Shelf Life | ... Evaporates rapidly in air, very unstable. |
| Decomposition | Decomposes in light ... . |
| Other Experimental Properties | Very unstable, volatile ... explodes above 60 °C. MP: less than or equal to -40 °C. BP: less than or equal to 71 °C |
| Chemical Classes | Nitrogen Compounds -> Other Nitrogen Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 120.36 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 118.909632 g/mol |
| Monoisotopic Mass | 118.909632 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
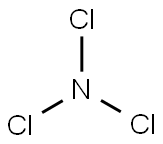
Nitrogen trichloride is a nitrogen halide.