100-39-0
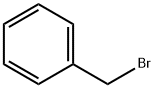
Product Name:
Benzyl bromide
Formula:
C7H7Br
Synonyms:
α-Bromotoluene;Benzyl bromide
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Benzyl bromide appears as a colorless liquid with an agreeable odor. Toxic by inhalation and by skin absorption. It is slightly soluble in water and denser than water (density 1.44 g / cm3 (Aldrich)). A lachrymator. Corrosive to metals and tissue. |
|---|---|
| Color/Form | Clear, refractive liquid |
| Odor | Very sharp; pungent; like tear gas |
| Boiling Point | 388 to 390 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 27 to 30 °F (NTP, 1992) |
| Flash Point | 188 °F (NTP, 1992) |
| Solubility | Reaction (NTP, 1992) |
| Density | 1.441 at 71.6 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 5.8 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 1 mmHg at 90 °F (NTP, 1992) |
| LogP | log Kow = 2.92 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | The substance decomposes slowly on contact with water producing hydrogen bromide. |
| Corrosivity | Corrosive to metal ... . |
| Heat of Vaporization | 120 Btu/lb = 66.4 cal/g = 2.78x10+5 J/kg |
| Surface Tension | 32.3 dynes/cm= 0.0323 N/m @ 20 °C |
| Refractive Index | Index of refraction: 1.5752 at 20 °C/D |
| Kovats Retention Index | 1087 1059 1061 |
| Other Experimental Properties | Lachrymator |
| Chemical Classes | Other Classes -> Halogenated Monoaromatics |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 171.03 g/mol |
|---|---|
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 1 |
| Exact Mass | 169.97311 g/mol |
| Monoisotopic Mass | 169.97311 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 55.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Benzyl bromide appears as a colorless liquid with an agreeable odor. Toxic by inhalation and by skin absorption. It is slightly soluble in water and denser than water (density 1.44 g / cm3 (Aldrich)). A lachrymator. Corrosive to metals and tissue.
