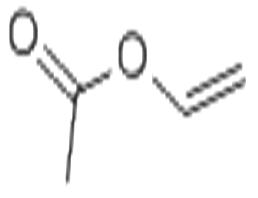

Vinyl acetate Monomer
| Price | USD1.50 | USD1.20 |
| Packge | 1KG | 5KG |
- Min. Order:500KG
- Supply Ability:100MT/Month Or Customized
- Time:2019-07-06
Product Details
- Product NameVinyl acetate Monomer
- CAS No.108-05-4
- EINECS No.203-545-4
- MFC4H6O2
- MW86.09
- InChIKeyXTXRWKRVRITETP-UHFFFAOYSA-N
- AppearanceLiquidClear colorless to almost colorless
- density 0.934 g/mL at 25 °C (lit.)
- Water Solubility 23 g/L (20 ºC)
- Melting point -93 °C (lit.)
- Boiling point 72-73 °C (lit.)
- storage temp. 2-8°C
SP032
| Vinyl acetate Monomer Basic information |
| Product Name: | Vinyl acetate Monomer |
| Synonyms: | 1-Acetoxyethylene;Acetate de vinyle;acetatedevinyle;acetatedevinyle(french);Acetic acid, ethylene ether;Aceticacid,ethenylester;aceticacid,ethyleneester;aceticacid,ethyleneether |
| CAS: | 108-05-4 |
| MF: | C4H6O2 |
| MW: | 86.09 |
| EINECS: | 203-545-4 |
| Product Categories: | Vinyl Esters;Building Blocks;C2 to C5;Carbonyl Compounds;Chemical Synthesis;Esters;Materials Science;Monomers;Organic Building Blocks;Polymer Science;VAM |
| Mol File: | 108-05-4.mol |
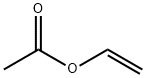 |
|
| Vinyl acetate Monomer Chemical Properties |
| Melting point | -93 °C |
| Boiling point | 72-73 °C(lit.) |
| density | 0.934 g/mL at 25 °C(lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 88 mm Hg ( 20 °C) |
| refractive index | n20/D 1.395(lit.) |
| Fp | 20 °F |
| storage temp. | 0-6°C |
| solubility | 20g/l |
| form | Liquid |
| color | Clear colorless to almost colorless |
| PH | 7 (20g/l, H2O, 20℃) |
| explosive limit | 2.6-13.4%(V) |
| Water Solubility | 23 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,9992 |
| BRN | 1209327 |
| Stability: | Stable. Highly flammable. Incompatible with acids, bases, oxidizing agents, peroxides, chlorosulfonic acid, ethylene imine, hydrochloric acid, oleum, nitric acid, sulfuric acid, 2-aminoethanol, light. Susceptible to polymerization; commercial product may be stabilized by the addition of hydroquinone. |
| CAS DataBase Reference | 108-05-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid ethenyl ester(108-05-4) |
| EPA Substance Registry System | Acetic acid ethenyl ester(108-05-4) |
| Safety Information |
| Hazard Codes | F,T,Xn |
| Risk Statements | 11-39/23/24/25-23/24/25-36-20/21/22-40-37-20 |
| Safety Statements | 16-23-29-33-45-36/37-7-9 |
| RIDADR | UN 1301 3/PG 2 |
| WGK Germany | 2 |
| RTECS | AK0875000 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29153200 |
| HS Code | 29333999 |
| Hazardous Substances Data | 108-05-4(Hazardous Substances Data) |
| Vinyl acetate Monomer Usage And Synthesis |
| Chemical Properties | colourless mobile liquid with a sweet, irritating odour |
| Uses | In polymerized form for plastic masses, films and lacquers; in plastic film for food packaging. As modifier for food starch. |
| General Description | A clear colorless liquid. Flash point 18°F. Density 7.8 lb / gal. Slightly soluble in water. Vapors are heavier than air. Vapors irritate the eyes and respiratory system. May polymerize if heated or contaminated. If polymerization occurs inside a container, the container may violently rupture. Used to make adhesives, paints, and plastics. |
| Air & Water Reactions | Highly flammable. Slightly soluble in water. |
| Reactivity Profile | Vinyl acetate may undergo spontaneous exothermic polymerization on exposure to light. Reacts with air or water to produces peroxides that initiate explosively violent polymerization. Reacts with hydrogen peroxide to form explosive peracetic acid. Reacts with oxygen to form explosive peroxides. Forms explosive Vinyl acetate ozonide on contact with ozone. Undergoes violent or explosive reactions with 2-aminoethanol, chlorosulfonic acid, ethylenediamine, mineral acids (hydrochloric acid, hydrofluoric acid, nitric acid, sulfuric acid, oleum), and peroxides [Lewis, 3rd ed., 1993, p. 1311]. Polymerization initiated by dibenzoyl peroxide in ethyl acetate accelerated out of control, ignited and exploded [Vervalin, 1973, p. 81]. Polymerization in toluene solution has caused several large industrial explosions [MCA Case History No. 2087]. |
| Health Hazard | Vinyl acetate has been related to reproductive abnormalities. It is a skin and upper respiratory tract irritantand a central nervous system depressant. Exposure caused gradual deterioration of heart muscles. |
| Fire Hazard | When heated to decomposition, Vinyl acetate burns and emits acrid fumes. Highly dangerous when exposed to heat, flames or oxidizers; explosion hazard with strong acids and strong oxidizers. Incompatible with alumina, oxidizing materials, 2-aminoethanol, chlorosulfonic acid; ethyleneimine; 36% hydrochloric acid; 48.7% hydrofluoric acid; 70% nitric acid; oleum; 96% sulfuric acid; ethylene diamine; peroxides and silica gel. Avoid light or any polymerizing initiator. Hazardous polymerization can be initiated by organic and inorganic peroxides; azo compounds; redox systems (including organometallic components); light; and high energy radiation. |
| Vinyl acetate Preparation Products And Raw materials |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP3年
- JSK Chemicals
- Vinyl acetate, 99% 108-05-4 99%
- Inquiry
- 2025-03-05
-
VIP0年
- JSK Chemicals
- Vinyl acetate, 99% 108-05-4 99%
- Inquiry
- 2025-03-05
- Mine Chem India
- Liquid Vinyl Acetate Monomer, Grade Standard: Reagent Grade
- Inquiry
- 2025-09-23
- Supreme India International
- Vinyl Acetate Monomer (VAM)
- Inquiry
- 2025-09-23
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY

