6153-56-6
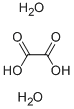
Product Name:
Oxalic acid dihydrate
Formula:
C2H6O6
Synonyms:
Ethanedioic acid;Ethanedioic acid dihydrate;Ethanedioic Acid, Dihydrate, Ethanedioic Acid;Oxalic acid dihydrate;Oxalic Acid, Dihydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder; Other Solid; Pellets or Large Crystals |
|---|---|
| Melting Point | 101-102 °C |
| Solubility | Solubility in water, g/100ml at 20 °C: 13-14 |
| Density | 1.65 g/cm³ |
| LogP | -0.81 |
| Chemical Classes | Other Classes -> Organic Acids |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 126.07 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 126.01643791 g/mol |
| Monoisotopic Mass | 126.01643791 g/mol |
| Topological Polar Surface Area | 76.6 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 71.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Oxalic acid dihydrate is used as a buffer for chromatographic separations and is used together with acetonitrile and/or other solvents for dechelation of metals and protein denaturation.
