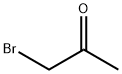
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Bromoacetone appears as a clear colorless liquid turning violet on standing, even in the absence of air, and decomposing to a black resinous mass on long standing. Denser than water and poorly soluble in water. Hence sinks in water. A violent lachrymator--low concentrations are very irritating to the eyes; high concentrations or prolonged exposure at lower concentrations may have adverse health effects. Very toxic by inhalation. Contact with the liquid causes painful burns. Used as a chemical war gas. |
|---|---|
| Color/Form | Colorless liquid; rapidly becomes violet even in absence of air |
| Odor | PUNGENT ODOR |
| Boiling Point | 277 °F at 760 mmHg (USCG, 1999) |
| Melting Point | -33.7 °F (USCG, 1999) |
| Flash Point | 124 °F (USCG, 1999) |
| Solubility | Soluble in ethanol, ether, and acetone |
| Density | 1.634 at 73.4 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 4.75 (Air = 1) |
| Vapor Pressure | 90.0 [mmHg] |
| LogP | 0.11 |
| Stability/Shelf Life | Turns violet rapidly even in absence of air. |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen bromide/. |
| Refractive Index | Index of refraction: 1.4697 at 15 °C/D |
| Dissociation Constants | pKa = 16.1 |
| Kovats Retention Index | 751.1 751 |
| Other Experimental Properties | Turns violet rapidly even in the absence of air |
| Chemical Classes | Toxic Gases & Vapors -> Other Toxic Gases & Vapors |
COMPUTED DESCRIPTORS
| Molecular Weight | 136.98 g/mol |
|---|---|
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 135.95238 g/mol |
| Monoisotopic Mass | 135.95238 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 42.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bromoacetone appears as a clear colorless liquid turning violet on standing, even in the absence of air, and decomposing to a black resinous mass on long standing. Denser than water and poorly soluble in water. Hence sinks in water. A violent lachrymator--low concentrations are very irritating to the eyes; high concentrations or prolonged exposure at lower concentrations may have adverse health effects. Very toxic by inhalation. Contact with the liquid causes painful burns. Used as a chemical war gas.