CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Viscous liquid |
| Melting Point | 228 °C |
| Solubility | 15 mg/L (at 24 °C) |
| LogP | 4.88 |
| LogS | -4.4 |
| Stability/Shelf Life | SENSITIVE TO LIGHT. |
| Decomposition | When heated to decomposition it emits very toxic fumes of sulfoxides, nitroxides, and /hydrogen chloride/. |
| Dissociation Constants | 8.1 |
| Collision Cross Section | 183.7 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 2937 2965 2970 2954 2970 2945.2 2980.7 2943 2997 2983 2921 2985 2954 2947.2 |
| Other Experimental Properties | Minute crystals; Solubility in water: less than 0.1% at 20 °C; slightly soluble in methanol, ethanol; practically insoluble in ether, benzene, chloroform; MP: 228 °C /Prochlorperazine dimaleate/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 373.9 g/mol |
|---|---|
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 373.1379466 g/mol |
| Monoisotopic Mass | 373.1379466 g/mol |
| Topological Polar Surface Area | 35 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 429 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
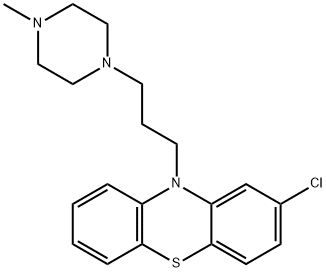
Prochlorperazine is a member of the class of phenothiazines that is 10H-phenothiazine having a chloro substituent at the 2-position and a 3-(4-methylpiperazin-1-yl)propyl group at the N-10 position. It has a role as an antiemetic, a dopaminergic antagonist, an alpha-adrenergic antagonist, a cholinergic antagonist, a first generation antipsychotic, an EC 3.4.21.26 (prolyl oligopeptidase) inhibitor and a dopamine receptor D2 antagonist. It is an organochlorine compound, a N-alkylpiperazine, a N-methylpiperazine and a member of phenothiazines. It derives from a hydride of a 10H-phenothiazine.