CHEMICAL AND PHYSICAL PROPERTIES
| Melting Point | Decomposes at 210-215 |
|---|---|
| Dissociation Constants | 7.435 |
| Collision Cross Section | 203.9 Ų [M+H]+ [CCS Type: TW] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 478.9 g/mol |
|---|---|
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 478.1142934 g/mol |
| Monoisotopic Mass | 478.1142934 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
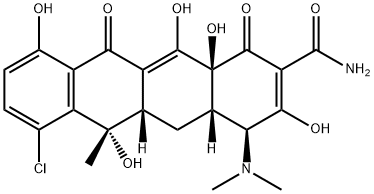
Chlortetracycline is a member of the class of tetracyclines with formula C22H23ClN2O8 isolated from Streptomyces aureofaciens. It has a role as an antiprotozoal drug, a fluorescent probe, a calcium ionophore and an antibacterial drug. It is a member of monochlorobenzenes, a tertiary amino compound, a tertiary alcohol, a monocarboxylic acid amide, a tertiary alpha-hydroxy ketone and a member of tetracyclines. It is a conjugate acid of a chlortetracycline(1-).