500-44-7
Product Name:
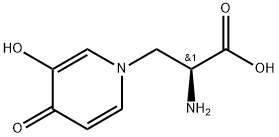
L-MIMOSINE
Formula:
C8H10N2O4
Synonyms:
(S)-α-Amino-β-[1-(3-hydroxy-4-oxopyridine)]propionic acid;Leucenol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | CRYSTALS FROM WATER |
| Melting Point | 228 dec °C |
| Solubility | SLIGHTLY SOL IN WATER; INSOL IN ALC, ETHER, ACETONE, BENZENE, DIOXANE, ACETIC ACID, ORDINARY ORGANIC SOLVENTS; SOL IN DILUTED ACETONE, DILUTED ALKALI |
| LogP | -2.5 |
| Optical Rotation | SPECIFIC OPTICAL ROTATION: -21 DEG @ 22 °C/D (WATER, C= 0.5); MAX ABSORPTION (WATER): 215 NM (LOG E= 4.6); 280 NM (LOG E= 4.6) |
| Collision Cross Section | 143.47 Ų [M+H]+ [CCS Type: DT, Method: stepped-field] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 198.18 g/mol |
|---|---|
| XLogP3 | -3.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 198.06405680 g/mol |
| Monoisotopic Mass | 198.06405680 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 321 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
L-mimosine is an L-alpha-amino acid that is propionic acid substituted by an amino group at position 2 and a 3-hydroxy-4-oxopyridin-1(4H)-yl group at position 3 (the 2S-stereoisomer). It a non-protein plant amino acid isolated from Mimosa pudica. It has a role as an EC 1.14.18.1 (tyrosinase) inhibitor and a plant metabolite. It is a non-proteinogenic L-alpha-amino acid and a member of 4-pyridones. It is functionally related to a propionic acid. It is a conjugate acid of a L-mimosine(1-). It is a tautomer of a L-mimosine zwitterion.
