CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Transparent solid; [Merck Index] Clear colorless liquid; [MSDSonline] Perfluoroalkanes are chemically stable, nonflammable, and physiologically inert; [Ullmann] |
|---|---|
| Color/Form | Course, transparent, needle-like crystals |
| Odor | Odorless |
| Boiling Point | 57.2 °C |
| Melting Point | -86.1 °C |
| Solubility | Soluble in ethyl ether, benzene, chloroform |
| Density | 1.6910 g/cu cm at 20 °C |
| Vapor Pressure | 218.0 [mmHg] |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Viscosity | 9.79 mP at 0 °C |
| Heat of Vaporization | Molar Heat of Vaporization (cal): 7307 at BP; 77.93 at 20 °C |
| Refractive Index | Index of refraction = 1.2515 at 20 °C/D |
| Other Experimental Properties | Global Warming Potential (GWP): Chemical: PFC-5-1-14 (Perfluorohexane, FC-72); GWP: 9,300 (100-Year Time Horizon) |
| Chemical Classes | Other Classes -> Halogenated Aliphatics, Saturated |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 338.04 g/mol |
|---|---|
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 3 |
| Exact Mass | 337.9776443 g/mol |
| Monoisotopic Mass | 337.9776443 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 319 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
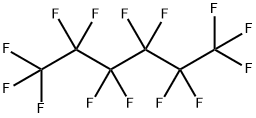
Perfluorohexane is a fluoroalkane that is hexane in which all of the hydrogens have been replaced by fluorines. It has a role as a radioopaque medium and a non-polar solvent. It is a fluorocarbon, a fluoroalkane and a volatile organic compound. It derives from a hydride of a hexane.