124937-52-6
Product Name:
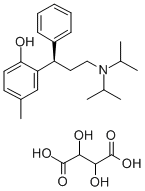
Tolterodine tartrate
Formula:
C26H37NO7
Synonyms:
E;s (R)-2-[3-[bis(1-methylethyl)-amino]1-phenylpropyl]-4-methylphenol [R-(R*,R*)]2,3dihydroxybutanedioate (1:1) (salt);(R)-Tolterodine L -tartrate;2-[(1R)-3-[Bis(1-methylethyl)amino]-1-phenylpropyl]-4-methyl-phenol tartrate;Detrusitol
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 475.6 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Exact Mass | 475.25700252 g/mol |
| Monoisotopic Mass | 475.25700252 g/mol |
| Topological Polar Surface Area | 139 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 474 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Tolterodine Tartrate is the tartrate salt form of tolterodine, a benzhydryl compound and a muscarinic receptor antagonist possessing both antimuscarinic and antispasmodic properties. Both tolterodine and its active metabolite, 5-hydroxymethyltolterodine, competitively blocks muscarinic receptors, thereby inhibiting acetylcholine receptor binding. This antagonistic action results in an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure, indicating an antimuscarinic action on the lower urinary tract.. The 5-hydroxymethyl metabolite appears to contribute significantly to the therapeutic effects.
