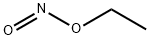CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ethyl nitrite, solution appears as a clear colorless to yellow liquid with a pleasant odor. Flammable over a wide range of vapor-air concentrations. Flash point -31 °F. Less dense than water and insoluble in water. Vapors are heavier than air and narcotic in high concentrations. Produces toxic oxides of nitrogen during combustion. |
|---|---|
| Color/Form | Colorless or yellowish, clear liquid |
| Odor | SWEET, RUM-LIKE ODOR |
| Taste | FRUITY TASTE |
| Boiling Point | 63 °F at 760 mmHg (USCG, 1999) |
| Melting Point | -58 °F (USCG, 1999) |
| Flash Point | -31 °F (USCG, 1999) |
| Solubility | Slightly soluble in water; decomposes in water; miscible with alcohol, ether |
| Density | 0.9 at 59 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 2.6 |
| Vapor Pressure | 620.0 [mmHg] |
| Stability/Shelf Life | ON KEEPING, IT GRADUALLY DECOMPOSES, BECOMING ACID & OXIDES OF NITROGEN FORM. DECOMP IS HASTENED BY AIR, LIGHT, & MOISTURE. |
| Autoignition Temperature | 194 °F (USCG, 1999) |
| Heat of Combustion | -7,800 BTU/LB= -4,300 CAL/G= -180X10+5 J/KG |
| Heat of Vaporization | 229 BTU/LB= 127 CAL/G= 5.32X10+5 J/KG |
| Surface Tension | 30 DYNES/CM= 0.030 N/M @ 20 °C |
| Refractive Index | Index of refraction: 1.3418 @ 10 °C/D |
| Kovats Retention Index | 377 |
| Other Experimental Properties | Decomp in water; decomp spontaneously at 194 °F (90 °C) |
| Chemical Classes | Nitrogen Compounds -> Nitrates and Nitrites |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 75.07 g/mol |
|---|---|
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 75.032028402 g/mol |
| Monoisotopic Mass | 75.032028402 g/mol |
| Topological Polar Surface Area | 38.7 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 28.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ethyl nitrite, solution appears as a clear colorless to yellow liquid with a pleasant odor. Flammable over a wide range of vapor-air concentrations. Flash point -31 °F. Less dense than water and insoluble in water. Vapors are heavier than air and narcotic in high concentrations. Produces toxic oxides of nitrogen during combustion.