Zonisamide
Synonym(s):1,2-Benzisoxazole-3-methanesulfonamide;AD 810, CI 912;Zonisamide - CAS 68291-97-4 - Calbiochem
- CAS NO.:68291-97-4
- Empirical Formula: C8H8N2O3S
- Molecular Weight: 212.23
- MDL number: MFCD00865316
- EINECS: 614-395-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-08 14:33:09
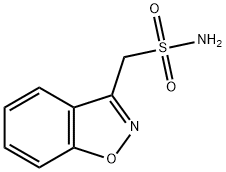
What is Zonisamide?
Absorption
Between 200 and 400 mg, zonisamide follows a dose-proportional pharmacokinetic profile. At concentrations higher than 800 mg, the Cmax and AUC increase in a disproportional manner, possibly due to zonisamide binding red blood cells. In healthy volunteers given 200 to 400 mg of zonisamide orally, peak plasma concentrations (Cmax) range between 2 and 5 μg/mL and are reached within 2–6 hours (Tmax). In healthy volunteers given 100 mg of zonisamide oral suspension, the Tmax ranged from 0.5 to 5 hours. Zonisamide has a high oral bioavailability (95%). The Tmax of zonisamide was delayed by food intake (4-6 hours); however, food has no effect on its bioavailability. Steady state is achieved 14 days after a stable dose is reached.
Toxicity
Information on daily doses over 800 mg/day of zonisamide is limited. During clinical development, three patients ingested unknown amounts of zonisamide as suicide attempts, and all of them were hospitalized with central nervous system symptoms. One patient became comatose and developed bradycardia, hypotension, and respiratory depression; 31 hours after zonisamide ingestion, plasma level was 100.1 μg/mL. Zonisamide plasma levels fell with a half-life of 57 hours, and the patient became alert five days later. There are no specific antidotes for zonisamide overdosage. In case of a suspected recent overdose, emesis should be induced or gastric lavage performed with the usual precautions to protect the airway. General supportive care is indicated, including frequent monitoring of vital signs and close observation. Due to its long half-life and low protein binding, renal dialysis may be effective in treating zonisamide overdose; however, the effectiveness of this procedure has not been formally studied.
In vivo studies found no evidence of carcinogenicity at zonisamide doses equivalent to or higher than the maximum recommended human dose (MRHD). In an in vitro chromosomal aberration assay in CHL cells, zonisamide displayed mutagenicity. Signs of reproductive toxicity were also detected in rats treated with a dose 0.5 times the MRHD.
Description
Zonisamide is a broad-spectrum antiepileptic effective in the treatment of refractory seizures. In cultured spinal cord neurons, zonisamide blocks the sustained firing of action potentials induced by depolarizing steps of current injected across the membrane.
The Uses of Zonisamide
anticonvulsant;carbonic anhydrase inhibitor, repetitive firing of voltage-gated sodium channels and reduction of T-type calcium channel currents blocker
Background
Zonisamide is a sulfonamide anticonvulsant used as an adjunctive therapy in adults with partial-onset seizures. Zonisamide may act by blocking repetitive firing of voltage-gated sodium channels, leading to a reduction of T-type calcium channel currents or by binding allosterically to GABA receptors. This latter action may inhibit the uptake of the inhibitory neurotransmitter GABA while enhancing the uptake of the excitatory neurotransmitter glutamate. Zonisamide represents an alternative for patients that remain refractory to established antiepileptic drugs. In 1989, it was approved for commercial use in Japan. The US and Europe approved it in 2000 and 2005, respectively.
Indications
Zonisamide capsules are indicated as adjunctive therapy in the treatment of partial seizures in adults with epilepsy. Zonisamide oral suspension is indicated as adjunctive therapy for the treatment of partial-onset seizures in adults and pediatric patients 16 years of age and older.
What are the applications of Application
1,2-benzoxazol-3-ylmethanesulfonamide is an anticonvulsant blocker of voltage-sensitive T-type Ca2+ and Na+ channels
Pharmacokinetics
By stopping the spread of seizure discharges, zonisamide prevents the extensor component of tonic convulsion, restricts the spread of focal seizures and prevents the propagation of seizures from the cortex to subcortical structures. In animal models, zonisamide was effective against tonic extension seizures but ineffective against clonic seizures. It also increased the threshold for generalized seizures and reduced the duration of cortical focal seizures. Aside from its antiepileptic effects, zonisamide is capable of activating neuroprotective mechanisms. It inhibits nitric oxide synthase and ??reduces ischemia-induced memory impairment and lipid peroxidation.
The use of zonisamide may lead to potentially fatal reactions. Severe reactions such as Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, and aplastic anemia have been reported in patients treated with sulfonamides such as zonisamide. Zonisamide may also lead to the development of serious hematological events, drug reaction with eosinophilia and systemic symptoms (DRESS) and multi-organ hypersensitivity, acute myopia and secondary angle closure glaucoma, as well as suicidal behaviour and ideation. Zonisamide is a carbonic anhydrase inhibitor, which may lead to metabolic acidosis in patients treated with this drug. Its therapeutic effects due to this pharmacological action are unknown.
Metabolism
Zonisamide metabolites are generated mainly by principally reductive and conjugative mechanisms. Oxidation reactions play a minor role in the metabolism of zonisamide. Zonisamide is metabolized by N-acetyl-transferases to form N-acetyl zonisamide and reduced to form the open ring metabolite, 2–sulfamoylacetylphenol (SMAP). The reduction of zonisamide to SMAP is mediated by CYP3A4. Zonisamide does not induce liver enzymes or its own metabolism.
Properties of Zonisamide
| Melting point: | 275°C dec. |
| Boiling point: | 223°C (rough estimate) |
| Density | 1.4306 (rough estimate) |
| Flash point: | 9℃ |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | H2O: >5 mg/mL, soluble |
| form | solid |
| color | off-white |
Safety information for Zonisamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Zonisamide
| InChIKey | UBQNRHZMVUUOMG-UHFFFAOYSA-N |
Abamectin manufacturer
KARPSCHEM LABORATORIES PVT. LTD.
Vercali Pharma
Ralington Pharma
Alfa Omega Pharma
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE) 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THFRelated products of tetrahydrofuran
You may like
-
 68291-97-4 Zonisamide 1,2-Benzisoxazole-3-methanesulfonamide 96%View Details
68291-97-4 Zonisamide 1,2-Benzisoxazole-3-methanesulfonamide 96%View Details
68291-97-4 -
 Zonisamide 98%View Details
Zonisamide 98%View Details
68291-97-4 -
 68291-97-4 Zonisamide 98%View Details
68291-97-4 Zonisamide 98%View Details
68291-97-4 -
 68291-97-4 98%View Details
68291-97-4 98%View Details
68291-97-4 -
 Zonisamide 68291-97-4 98%View Details
Zonisamide 68291-97-4 98%View Details
68291-97-4 -
 68291-97-4 Zonisamide 98%View Details
68291-97-4 Zonisamide 98%View Details
68291-97-4 -
 Zonisamide 98%View Details
Zonisamide 98%View Details
68291-97-4 -
 68291-97-4 Zonisamide 98%View Details
68291-97-4 Zonisamide 98%View Details
68291-97-4