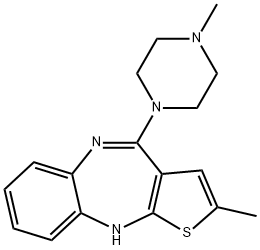
Olanzapine
Synonym(s):2-Methyl-4-(4-methyl-1-piperazinyl)- 10H-thieno[2,3-b][1,5]benzodiazepine;LY-170053;Olanzapine
- CAS NO.:132539-06-1
- Empirical Formula: C17H20N4S
- Molecular Weight: 312.43
- MDL number: MFCD00866702
- EINECS: 603-618-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-09 17:37:02
What is Olanzapine?
Absorption
Olanzapine presents a linear pharmacokinetic profile and, after daily administration, it reaches steady-state in about a week. Under the administration of a normal dosage of olanzapine, the steady-state plasma concentration does not seem to exceed 150 ng/ml with an AUC of 333 ng/h/ml.
The absorption of olanzapine is not affected by the concomitant administration of food. The pharmacokinetic profile of olanzapine is characterized by reaching peak plasma concentration of 156.9 ng/ml approximately 6 hours after oral administration.
Toxicity
The toxicity symptoms of olanzapine are known to include somnolence, mydriasis, blurred vision, respiratory depression, hypotension, extrapyramidal symptoms and anticholinergic effects. The overdosage effects in children are generally associated with more significant side effects.
The maximum registered dosage of olanzapine in clinical trials was of 300 mg and it was reported to present drowsiness and slurred speech. However, on post-marketing surveillance, a wide range of symptoms have been presented including agitation, dysarthria, tachycardia, extrapyramidal symptoms, and reduced consciousness. One case of overdosage-driven death was reported after ingestion of 450 mg of olanzapine. In the cases of acute overdosage, the establishment of adequate oxygenation and ventilation, gastric lavage and administration of activated charcoal with a laxative is recommended.
In carcinogenesis studies, olanzapine was showed to present an increase in the incidence of liver hemangiomas and hemangiosarcomas as well as mammary gland adenomas, and adenocarcinomas. On fertility studies, there was solely found impairment in male mating performance and delays in ovulation. There is no evidence of mutagenic, genotoxic potential not adverse events on fertility.
Description
Zyprexa was launched in Canada, Germany, the UK and US as an antipsychotic agent. Prepared in three steps via the intermediate diazepinone, it is an atypical antipsychotic with a high affinity for dopaminergic and serotonergic receptors. Specifically, olazapine is a potent 5-HT2/D2 antagonist with anticholinergic activity. It has a greater antagonistic effect at 5-HT2a than at dopamine D2 receptors and in vivo is clozapine-like. Thus it is less likely to produce extrapyramidal side effects and does not produce any granulocytopenia. Its 10 metabolic products are all inactive.
Description
Olanzapine is a drug (Zyprexa) used to treat schizophrenia. In January 2007, the US Federal Circuit Court ruled that Eli Lilly''s olanzapine patent was valid and had been infringed by other drug makers.
The Uses of Olanzapine
A serotonin (5-HT2) and dopamine (D1/D2) receptor antagonist with anticholinergic activity. Antipsychotic.
Indications
Olanzapine was initially used orally and intramuscularly for the chronic treatment of schizophrenia in patients over 13 years old and other psychiatric disorders such as bipolar I disorder including mixed or manic episodes.
Olanzapine is also indicated, in combination with lithium or valproate for the short-term treatment of acute manic or mixed episodes associated with bipolar I disorder in adults.
As well, olanzapine is indicated, in combination with fluoxetine for the treatment of episodes of depression associated with bipolar disorder type 1 and treatment-resistant depression in patients over 10 years old.
Olanzapine is also approved for the management of psychomotor agitation associated with schizophrenia and bipolar I mania.
Schizophrenia is a complex biochemical brain disorder that affects the person's ability to differentiate reality. It is usually observed as the presence of delusions, hallucinations, social withdrawal and disturbed thinking.
Bipolar disorder is a mental health condition defined by periods of extreme mood disturbances. It is categorized in different types from which type 1 is known to involve episodes of severe mania and often depression while type 2 presents less severe forms of mania.
Olanzapine is also indicated in combination with samidorphan for the treatment of bipolar I disorder, either as an adjunct to lithium or valproate or as monotherapy for the acute treatment of manic or mixed episodes or as maintenance therapy, and for the treatment of schizophrenia in adults.
Background
Olanzapine is a thienobenzodiazepine classified as an atypical or second-generation antipsychotic agent. The second-generation antipsychotics were introduced in the 90s and quickly gained traction due to their impressive efficacy, reduced risk for extrapyramidal side effects and reduced susceptibility to drug-drug interactions. Olanzapine very closely resembles clozapine and only differs by two additional methyl groups and the absence of a chloride moiety. It was discovered by scientists at Eli Lilly and approved to be marketed in the US in 1996.
What are the applications of Application
Olanzapine is a D2DR and serotonin inhibitor
Pharmacokinetics
The effect of olanzapine in the D2 receptor is reported to produce the positive effects of this drug such as a decrease in hallucinations, delusions, disorganized speech, disorganized thought, and disorganized behavior. On the other hand, its effect on the serotonin 5HT2A receptor prevents the onset of anhedonia, flat affect, alogia, avolition and poor attention. Based on the specific mechanism of action, olanzapine presents a higher affinity for the dopamine D2 receptor when compared to the rest of the dopamine receptor isotypes. This characteristic significantly reduces the presence of side effects.
Clinical trials for the original use of olanzapine demonstrated significant effectiveness in the treatment of schizophrenia and bipolar disorder in adults and acute manic or mixed episodes associated with bipolar disorder in adolescents.
The effect of olanzapine on dopamine and serotonin receptors has been suggested to reduce chemotherapy-induced nausea and vomiting as those receptors are suggested to be involved in this process. For this effect, several clinical trials have been conducted and it has been shown that olanzapine can produce a significant increase in total control of nausea and vomiting. In a high-level study of the effect of olanzapine for this condition, a complete response on the delay phase was observed in 84% of the individual and control of emesis of over 80% despite the phase.
Metabolism
Olanzapine is greatly metabolized in the liver, which represents around 40% of the administered dose, mainly by the activity of glucuronide enzymes and by the cytochrome P450 system. From the CYP system, the main metabolic enzymes are CYP1A2 and CYP2D6. As part of the phase I metabolism, the major circulating metabolites of olanzapine, accounting for approximate 50-60% of this phase, are the 10-N-glucuronide and the 4'-N-desmethyl olanzapine which are clinically inactive and formed by the activity of CYP1A2. On the other hand, CYP2D6 catalyzes the formation of 2-OH olanzapine and the flavin-containing monooxygenase (FMO3) is responsible for N-oxide olanzapine.
On the phase II metabolism of olanzapine, UGT1A4 is the key player by generating direct conjugation forms of olanzapine.
Properties of Olanzapine
| Melting point: | 195°C |
| Boiling point: | 476.0±55.0 °C(Predicted) |
| Density | 1.32±0.1 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: >15mg/mL |
| form | powder |
| color | yellow |
Safety information for Olanzapine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Olanzapine
Abamectin manufacturer
Jigs Chemical ltd
GLP Pharma Standards
Rampex Labs Private Limited
Cadila Pharmaceuticals Ltd
Dr. Reddy's Laboratories Ltd
Vision Drugs Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine N-Benzyl-3-hydroxypiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran




You may like
-
 132539-06-1 98%View Details
132539-06-1 98%View Details
132539-06-1 -
 Olanzapine 132539-06-1 98%View Details
Olanzapine 132539-06-1 98%View Details
132539-06-1 -
 Olanzapine 99%View Details
Olanzapine 99%View Details
132539-06-1 -
 Olanzapine 98%View Details
Olanzapine 98%View Details
132539-06-1 -
 Olanzapine 99%View Details
Olanzapine 99%View Details
132539-06-1 -
 Olanzapine 98%View Details
Olanzapine 98%View Details -
 Olanzapine 132539-06-1 98%View Details
Olanzapine 132539-06-1 98%View Details
132539-06-1 -
 Olanzapine 132539-06-1 99%View Details
Olanzapine 132539-06-1 99%View Details
132539-06-1