Lenalidomide
Synonym(s):Lenalidomide;3-(4-Amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione;3-(4-Amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione;3-(4-Amino-1-oxoisoindolin-2-yl)piperidin-2,6-dione;1-Oxo-4-amino-2-(2,6-dioxopiperidin-3-yl)isoindole
- CAS NO.:191732-72-6
- Empirical Formula: C13H13N3O3
- Molecular Weight: 259.26
- MDL number: MFCD18064659
- EINECS: 691-297-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-13 18:00:15
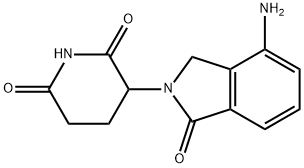
What is Lenalidomide ?
Description
Lenalidomide is a kind of antitumor drugs that developed by American biological pharmaceutical companies. Its chemical structure is similar with thalidomide. It differing in the presence of an amino moiety in the 4-position and removal of one of the carbonyls of the phthaloyl ring. This derivative evolved from a structural-based effort to eliminate the adverse effects (somnolence, neuropathy, and teratogenicity) of thalidomide while maintaining or enhancing the appealing attributes. It has many functions such as anti-tumor, immune regulation and anti-angiogenesis. It can inhibit the secretion of inflammatory cytokines, and increase the secretion of peripheral blood mononuclear anti-inflammatory cytokines. Vitro tests show that lenalidomide can inhibit the hyperplasia of some cell lines such as namalwa cell. It can inhibit the growth of patients’ multiple myeloma cells and MM1S cell. In addition, lenalidomide also can inhibit the expression of oxidase-2 (COX-2), but it has no effect on COX-1. Two multicenter randomized double-blind placebo-controlled clinical studies evaluate the safety and curative effect of lenalidomide that is used for multiple myeloma. The primary efficacy end point of the studies is time to progression (TTP). The interim analysis shows that TTP of the combination group is significantly superior to dexamethasone group. Recent clinical research results show that lenalidomide not only has curative effect on treating MDS and MM, but also on treating myeloma, leukemia, metastatic renal cell carcinoma, solid tumor, idiopathic generalized amyloidosis and systemic bone marrow fibrosis disease with marrow unripe.
The Uses of Lenalidomide
Lenalidomide (Revlimid, CC-5013) is a TNF-α secretion inhibitor with IC50 of 13 nM.
Indications
Lenalidomide is indicated for the treatment of adult patients with multiple myeloma (MM) in combination with dexamethasone. It is also indicated as maintenance therapy in multiple myeloma following autologous hematopoietic stem cell transplantation (auto-HSCT).
It is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities.
Lenalidomide is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib.
In combination with a rituximab product, lenalidomide is indicated for the treatment of adult patients with previously treated follicular lymphoma (FL) or previously treated marginal zone lymphoma (MZL).
Background
Lenalidomide (previously referred to as CC-5013) is an immunomodulatory drug with potent antineoplastic, anti-angiogenic, and anti-inflammatory properties. It is a 4-amino-glutamyl analogue of thalidomide and like thalidomide, lenalidomide exists as a racemic mixture of the active S(-) and R(+) forms. However, lenalidomide is much safer and potent than thalidomide, with fewer adverse effects and toxicities. Thalidomide and its analogues, including lenalidomide, are referred to as immunomodulatory imide drugs (also known as cereblon modulators), which are a class of immunomodulatory drugs that contain an imide group. Lenalidomide works through various mechanisms of actions that promote malignant cell death and enhance host immunity. Available as oral capsules, lenalidomide is approved by the FDA and EU for the treatment of multiple myeloma, myelodysplastic syndromes, mantle cell lymphoma, follicular lymphoma, and marginal zone lymphoma in selected patients. Due to severe teratogenicity, pregnancy must be excluded before the start of treatment and patients must enroll in the lenalidomide Risk Evaluation and Mitigation Strategy (REMS) program to ensure contraception adherence.
Pharmacokinetics
In hematological malignancies, the immune system is deregulated in the form of altered cytokine networks in the tumour microenvironment, defective T cell regulation of host-tumour immune interactions, and diminished NK cell activity. Lenalidomide is an immunomodulatory agent with antineoplastic, antiangiogenic, and anti-inflammatory properties. Lenalidomide exerts direct cytotoxicity by increasing apoptosis and inhibiting the proliferation of hematopoietic malignant cells. It delays tumour growth in nonclinical hematopoietic tumour models in vivo, including multiple myeloma. Lenalidomide also works to limit the invasion or metastasis of tumour cells and inhibits angiogenesis.
Lenalidomide also mediates indirect antitumour effects via its immunomodulatory actions: it inhibits the production of pro-inflammatory cytokines, which are implicated in various hematologic malignancies. Lenalidomide enhances the host immunity by stimulating T cell proliferation and enhancing the activity of natural killer (NK) cells. Lenalidomide is about 100–1000 times more potent in stimulating T cell proliferation than thalidomide. In vitro, it enhances antibody-dependent cell-mediated cytotoxicity (ADCC), which is even more pronounced when used in combination with rituximab. Due to its anti-inflammatory properties, lenalidomide has been investigated in the context of inflammatory and autoimmune diseases, such as amyotrophic lateral sclerosis.
Absorption
Following oral administration, lenalidomide is rapidly absorbed with high bioavailability. It has a Tmax ranging from 0.5 to six hours. Lenalidomide exhibits a linear pharmacokinetic profile, with its AUC and Cmax increasing proportionally with dose. Multiple dosing does not result in drug accumulation. In healthy male subjects, the Cmax was 413 ± 77 ng/ml and the AUCinfinity was 1319 ± 162 h x ng/ml.
Metabolism
Lenalidomide is not subject to extensive hepatic metabolism involving CYP enzymes and metabolism contributes to a very minor extent to the clearance of lenalidomide in humans. Lenalidomide undergoes hydrolysis in human plasma to form 5-hydroxy-lenalidomide and N-acetyl-lenalidomide. Unchanged lenalidomide is the predominant circulating drug form, with metabolites accounting for less than five percent of the parent drug levels in the circulation.
Toxicity
The lowest lethal dose (LDLo) in rats is >2000 mg/kg following oral administration and >40 mg/kg following intravenous administration. The oral Lowest published toxic dose (TDLo) in humans is 9 mg/kg/4W (intermittent).
There is limited clinical experience in managing lenalidomide overdose. In single-dose studies, healthy subjects have been exposed to doses up to 400 mg. In clinical trials, the dose-limiting toxicity was neutropenia and thrombocytopenia. Toxicities associated with lenalidomide, some leading to fatality, include embryo-fetal toxicity, neutropenia, thrombocytopenia, venous (deep vein thrombosis and pulmonary embolism) and arterial thromboembolic events (myocardial infarction and stroke), serious adverse cardiovascular reactions, second primary malignancies, hepatotoxicity, severe cutaneous reactions, tumour lysis syndrome, tumour flare reaction, hypothyroidism, and hyperthyroidism.
Properties of Lenalidomide
| Melting point: | 265-268 °C |
| Boiling point: | 614.0±55.0 °C(Predicted) |
| Density | 1.460±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 30 mg/ml) |
| form | solid |
| color | White |
Safety information for Lenalidomide
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Lenalidomide
| InChIKey | GOTYRUGSSMKFNF-UHFFFAOYSA-N |
| SMILES | N1C(=O)CCC(N2CC3=C(C2=O)C=CC=C3N)C1=O |
Abamectin manufacturer
Jigs Chemical ltd
MANPICK LABORATORIES PVT LTD
Dr. Reddy's Laboratories Ltd
Bulat Pharmaceutical Pvt Ltd
KARPSCHEM LABORATORIES PVT. LTD.
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran


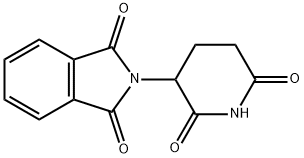
You may like
-
 191732-72-6 >98%View Details
191732-72-6 >98%View Details
191732-72-6 -
 Lenalidomide (Form A) 98%View Details
Lenalidomide (Form A) 98%View Details
191732-72-6 -
 191732-72-6 98%View Details
191732-72-6 98%View Details
191732-72-6 -
 191732-72-6 Lenalidomide 99%View Details
191732-72-6 Lenalidomide 99%View Details
191732-72-6 -
 191732-72-6 98%View Details
191732-72-6 98%View Details
191732-72-6 -
 191732-72-6 Lenalidomide 98%View Details
191732-72-6 Lenalidomide 98%View Details
191732-72-6 -
 Lenalidomide 98%View Details
Lenalidomide 98%View Details
191732-72-6 -
 Lenalidomide 191732-72-6 99%View Details
Lenalidomide 191732-72-6 99%View Details
191732-72-6