Azithromycin
Synonym(s):N-Methyl-11-aza-10-deoxo-10-dihydroerythromycin A;Azithromycin;Azithromycin dihydrate;CP 62,993;CP-62993
- CAS NO.:83905-01-5
- Empirical Formula: C38H72N2O12
- Molecular Weight: 748.98
- MDL number: MFCD00873574
- EINECS: 617-500-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-06-06 16:20:44
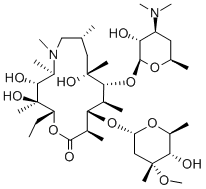
What is Azithromycin?
Absorption
Bioavailability of azithromycin is 37% following oral administration. Absorption is not affected by food. Macrolide absorption in the intestines is believed to be mediated by P-glycoprotein (ABCB1) efflux transporters, which are known to be encoded by the ABCB1 gene .
Toxicity
Possible major adverse effects include cardiovascular arrhythmias and hearing loss. Macrolide resistance is also an ongoing issue. Hepatotoxicity has been observed in rare cases.
Due to the act that azithromycin is mainly eliminated by the liver, caution should be observed when azithromycin is given to patients with decreased hepatic function.
Because limited data in patients with renal GFR<10 mL/min, caution should be exercised when prescribing azithromycin to these patients.
Use in Pregnancy: Azithromycin is categorized as a pregnancy category B drug. It should be used during pregnancy only if clearly needed.
Nursing Mothers: Because many other drugs are excreted in human milk, caution should be observed when azithromycin is given to a nursing woman.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies in animals have not been performed to study carcinogenic potential. Azithromycin has demonstrated no potential to be mutagenic in standard laboratory tests. No evidence of negative effects on fertility due to azithromycin was found.
Description
Azithromycin was the first of the azalides and was designed to improve the stability and biological half-life of erythromycin A, as well as improve activity against Gram negative bacteria. Azithromycin is a long-acting macrolide antibiotic structurally related to erythromycin A (EA), having a methyl-substituted nitrogen at position 9a in the aglycone ring.
Background
Azithromycin is a broad-spectrum macrolide antibiotic with a long half-life and a high degree of tissue penetration . It was initially approved by the FDA in 1991.
It is primarily used for the treatment of respiratory, enteric and genitourinary infections and may be used instead of other macrolides for some sexually transmitted and enteric infections.
In March 2020, a small study was funded by the French government to investigate the treatment of COVID-19 with a combination of azithromycin and the anti-malaria drug hydroxychloroquine. The results were positive, all patients taking the combination were virologically cured within 6 days of treatment, however, larger studies are required.
Indications
Azithromycin is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the microorganisms listed in the specific conditions below. Recommended dosages, duration of therapy and considerations for various patient populations may vary among these infections.
Adults:
Acute bacterial exacerbations of chronic obstructive pulmonary disease due to Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae
Acute bacterial sinusitis due to Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae
Community-acquired pneumonia due to Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae or Streptococcus pneumoniae in patients appropriate for oral therapy
Pharyngitis/tonsillitis caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy. Uncomplicated skin and skin structure infections due to Staphylococcus aureus, Streptococcus pyogenes, or Streptococcus agalactiae. Abscesses usually require surgical drainage.
Urethritis and cervicitis due to Chlamydia trachomatis or Neisseria gonorrhoeae.
Genital ulcer disease in men due to Haemophilus ducreyi (chancroid). Due to the small number of women included in clinical trials, the efficacy of azithromycin in the treatment of chancroid in women has not been established.
Pharmacokinetics
Macrolides stop bacterial growth by inhibiting protein synthesis and translation, treating bacterial infections . Azithromycin has additional immunomodulatory effects and has been used in chronic respiratory inflammatory diseases for this purpose .
Structure
Azithromycin is structurally related to erythromycin. Azithromycin [9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin] is a part of the azalide subclass of macrolides, and contains a 15-membered ring, with a methyl-substituted nitrogen instead of a carbonyl group at the 9a position on the aglycone ring, which allows for the prevention of its metabolism. This differentiates azithromycin from other types of macrolides.
Metabolism
In vitro and in vivo studies to assess the metabolism of azithromycin have not been performed , however, this drug is eliminated by the liver , .
Properties of Azithromycin
| Melting point: | 113-115°C |
| Boiling point: | 822.1±65.0 °C(Predicted) |
| Density | 1.18±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Practically insoluble in water, freely soluble in anhydrous ethanol and in methylene chloride. |
| form | neat |
| color | White to Off-White |
Safety information for Azithromycin
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Azithromycin
Abamectin manufacturer
CHEMXTREE STANDARDS
SNECOFRi PVT LTD
Shree HariKrishna Pharmaceuticals
Varanous Labs Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine N-Benzyl-3-hydroxypiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran






You may like
-
 83905-01-5 Azithromycin 98%View Details
83905-01-5 Azithromycin 98%View Details
83905-01-5 -
 83905-01-5 98%View Details
83905-01-5 98%View Details
83905-01-5 -
 Azithromycin 98%View Details
Azithromycin 98%View Details -
 AZITHROMYCIN 99%View Details
AZITHROMYCIN 99%View Details -
 Azithromycin 83905-01-05 98%View Details
Azithromycin 83905-01-05 98%View Details
83905-01-05 -
 Azithromycin 99%View Details
Azithromycin 99%View Details -
 83905-01-5 98%View Details
83905-01-5 98%View Details
83905-01-5 -
 Azithromycin 99%View Details
Azithromycin 99%View Details