1,2,4-Triazole
Synonym(s):1,2,4-Triazole
- CAS NO.:288-88-0
- Empirical Formula: C2H3N3
- Molecular Weight: 69.07
- MDL number: MFCD00005228
- EINECS: 206-022-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-24 21:11:45
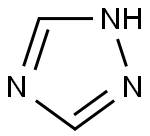
What is 1,2,4-Triazole?
Chemical properties
white crystalline powder and flakes
Description
1,2,4-Triazole is composed of two pyridine atoms and a pyrrole nitrogen atom. It is inactive to electrophilic attack and thus resembles pyridine. Simple triazoles do not undergo nitration, sulfonation or N-oxidation. However, the triazolol anion reacts readily with electrophiles, and alkylation and acylation reactions are the most studied reactions.
The Uses of 1,2,4-Triazole
1,2,4-Triazole is present in a large variety of fungicides as C14-demethylase-inhibitors. In addition they are used in synthetic chemical reactions such as the design and preparation of ghrelin receptor ligand antagonists, like JMV 2959.
The Uses of 1,2,4-Triazole
1,2,4-Triazole is used in the production of pesticides, pharmaceuticals (fluconazole), dyes and rubber additives, and also in the photoconductor of replication system. Intermediate of antifungal fluconazole.
The Uses of 1,2,4-Triazole
1,2,4-triazole and its derivatives are important structural moieties of many pharmaceutical drugs. Triazoles can also act as ligands to form coordination complexes with transition metal ions. Due to their electron-deficient nature, they exhibit excellent electron-transport and hole-blocking properties, making them promising organic materials in material science applications.
What are the applications of Application
1,2,4-Triazole is A chemical present in a variety of fungicides
Definition
A heterocyclic organic compound with a five-membered ring containing two carbon atoms and three nitrogen atoms. There are two isomers.
Definition
1,2,4-triazole is a chemical substance with molecular formula C2H3N3, molecular weight 69.07 and CAS No. 288-88-0. Colorless acicular crystal, soluble in water and ethanol. 1H-1,2,4-triazole is a 1,2,4-triazole. It is a tautomer of a 3H-1,2,4-triazole and a 4H-1,2,4-triazole.
Triazole is an aromatic five membered heterocyclic compound. Its unique structure is conducive to the formation of a variety of non covalent bonds and is easy to promote the binding of proteins, enzymes and receptors, thus showing a wide range of biological pharmacological activities, such as anticancer, antispasmodic, antibacterial, anti HIV. In particular, 1,2,3-triazole has strong thermal stability and acid-base stability, and is not sensitive to redox, hydrolysis and enzymatic degradation. It is often used as a pharmacodynamic group in drug synthesis.
Synthesis
1H-1,2,4-triazole is obtained by the hydrolysis of 1,2-bis(butan-2-ylidene)hydrazine with formamide at 170°C.
Flammability and Explosibility
Non flammable
Synthesis
Alkyl bromide and sodium azide were used as raw materials to prepare organic azides, and a series of 1,2,4-Triazole small molecules were prepared by click chemistry. The structures of the six products were characterized by 1H NMR, 13C NMR and IR. the products were expected targets. Furthermore, the effect of different substituent structure on the reaction was discussed. Especially the introduction of drug molecule ethisterone, which is expected to provide a certain experimental basis for the application of triazole and its derivatives in biomedicine and other fields. As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.
As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.
Purification Methods
Crystallise 1,2,4-triazole from EtOH, H2O, EtOAc (m 120.5-121o), or EtOH/*C6H6. The hydrochloride has m 170o, and the picrate has m 163-164o (from H2O or CHCl3). [Barszcz et al. J Chem Soc, Dalton Trans 2025 1986]. [Beilstein 26 H 13, 26 II 6, 26 III/IV 35.]
Properties of 1,2,4-Triazole
| Melting point: | 119-121 °C (lit.) |
| Boiling point: | 260 °C (lit.) |
| Density | 1.15 g/cm3 (130℃) |
| vapor pressure | 0.215Pa at 20℃ |
| refractive index | 1.4854 (estimate) |
| Flash point: | 140 °C |
| storage temp. | Store below +30°C. |
| solubility | 547g/l |
| form | Crystalline Powder and Flakes |
| pka | 2.27(at 20℃) |
| color | White |
| PH | 8 (10g/l, H2O, 20℃) |
| Water Solubility | 1250 g/L (20 ºC) |
| Merck | 14,9605 |
| BRN | 104767 |
| CAS DataBase Reference | 288-88-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-1,2,4-Triazole(288-88-0) |
| EPA Substance Registry System | 1,2,4-Triazole (288-88-0) |
Safety information for 1,2,4-Triazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 1,2,4-Triazole
| InChIKey | NSPMIYGKQJPBQR-UHFFFAOYSA-N |
Abamectin manufacturer
Chynops Pharma
RATNAMANI BIO CHEMICALS AND PHARMACEUTICALS PVT LTD
LANXESS India Pvt. Ltd.
Omega Finechem Private Limited
Sibram Pharmaceutical
New Products
Bromine 99.5% AR (4 x 500ml) Fehling's Solution No. B Amino Acid Kit of 23 items set Ammonium Molybdate Reagent Solution Beam's Reagent Solution Ehrlich's Reagent For detection of urobillinogen 4-Hydroxy Carbazole 4-((2-isopropoxyethoxy)methyl)phenol 2 – Methoxy – 5- Sulfamoyl Benzoic acid Amino Salicylic Acid. U.S.P. 1,2,3,4-Tetrahydrocarbazol-4-one Acetone Isobutryl oxime ester Curcuma aromatica Oil Curry leaf Extract Terminalia bellirica Extract Aloe vera extract 200x Withania somnifera (Ashwagandha Extract) Citrus bioflavonoids Extract (S)-4-(4-(5-(Aminomethyl)-2-Oxooxazolidin-3-Yl)Phenyl)Mo Ethyl N-[[2-[[[4-(Aminoiminomethyl)Phenyl]Amino]Methyl] 5-Nitrosalicylaldehyde 5-(Difluoromethoxy)-2-Mercapto-1H-Benzimidazole- IP/BP/ Cypermethric Acid Chloride Methyl Di Chloride (Mdc)Related products of tetrahydrofuran
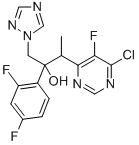

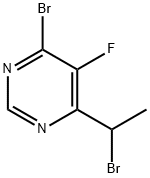

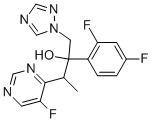
You may like
-
 1,2,4-Triazole 288-88-0 98%View Details
1,2,4-Triazole 288-88-0 98%View Details
288-88-0 -
 288-88-0 1,2,4-Triazole 99%View Details
288-88-0 1,2,4-Triazole 99%View Details
288-88-0 -
 1,2,4-Triazole pure CAS 288-88-0View Details
1,2,4-Triazole pure CAS 288-88-0View Details
288-88-0 -
 1,2,4-Triazole, 99% CAS 288-88-0View Details
1,2,4-Triazole, 99% CAS 288-88-0View Details
288-88-0 -
 288-88-0 98+View Details
288-88-0 98+View Details
288-88-0 -
 1,2,4-Triazole CAS 288-88-0View Details
1,2,4-Triazole CAS 288-88-0View Details
288-88-0 -
 1,2,4-Triazole CAS 288-88-0View Details
1,2,4-Triazole CAS 288-88-0View Details
288-88-0 -
 1,2,4-Triazole CAS 288-88-0View Details
1,2,4-Triazole CAS 288-88-0View Details
288-88-0